Cationic Polymerization
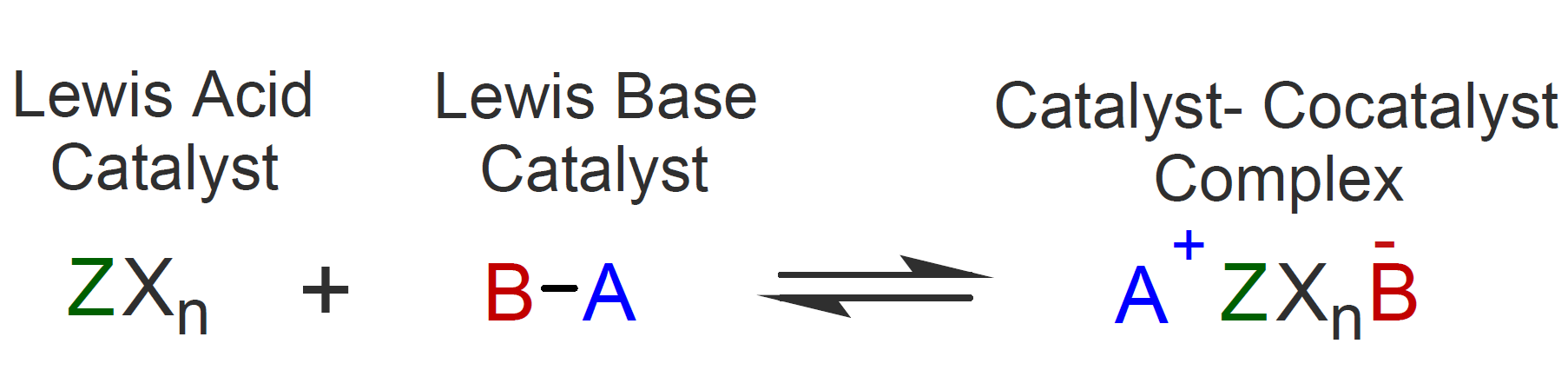
Many vinyl monomers with electron-releasing groups such as alkoxy, phenyl, or alkyl readily polymerize in the presence of very small amounts of a catalyst of the type used in Friedel-Crafts reactions. Examples of effective catalysts are AlCl3, AlBr3, BF3, TiCl4, SnCl4. Sometimes, strong protonic acids such as H2SO4, HClO4 or H3PO4 are also used.1,4 The Friedel-Craft catalysts are examples of Lewis acids with strong electron-acceptor capability.10 They usually require a co-catalyst, namely a Lewis base such as water, alcohol or acetic acid which forms a complex with the catalyst that stabilizes the counterion and prevents recombination.2,9 :
A common Lewis acid is boron trifluoride BF3 which, when reacting with trace amounts of water, forms an electrophile that can initate chain growth:
![]()
The initiator complex can exist in three different forms in the reaction mixture: (a) as ionized molecules, (b) as ion pairs or (c) as free (solvated) ions.5 The degree of aggregation and solvation will significantly affect the polymerization kinetics which depends on the solvation energies of the ions (which favors dissociation), and the attractive forces between the ions (which causes their association).6 In general, for dissociated ion pairs, the reaction rate is greater than for tight (solvent-bridged) ion pairs.5 This explains why the kinetik and rate of polymerization is strongly affected by the chemical nature of the solvent and the initiator complex, and not only by the type of monomer, the temperature and amount of initiator. In most cationic polymerization systems, the anions are (much) larger than the cations, so that the charge density is relatively small. Then the solvation of the anions can, to a first approximation, be neglected. On the other hand, the solvation of the carbocation can be rather strong.6,8
Similar to a free radical polymerization, the initiator adds to a monomer which creates the propagating chain growth center. In the case of a Lewis acid-proton donor complex such as boron trifluoride monohydride, a proton is transfered from the complex to the double bond of the vinyl monomer which produces a reactive carbocation:
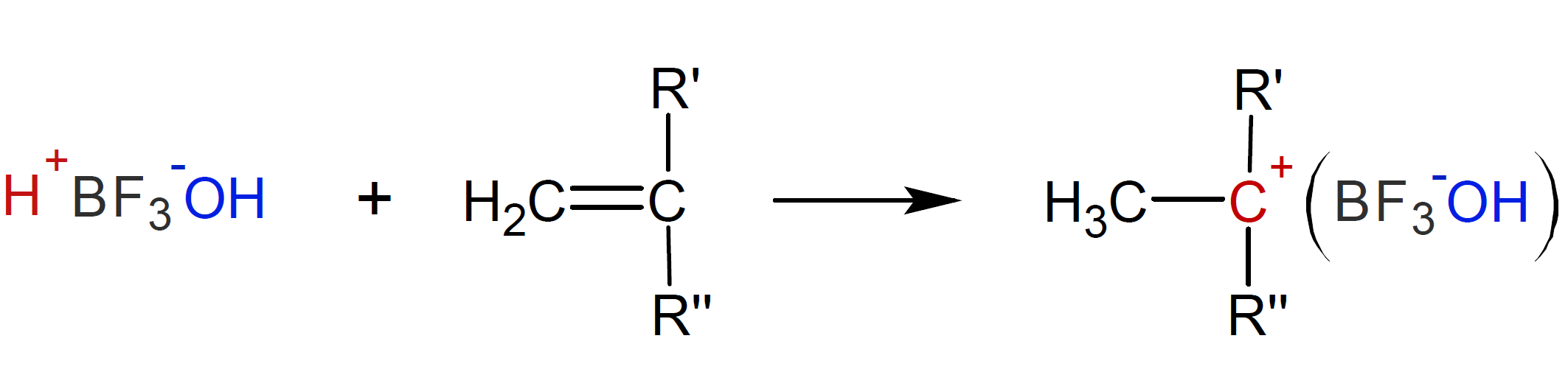
where R' is a electron-releasing substituent and R'' is a hydrogen or another electron-releasing
substituent such as an alkoxy or alkyl group.
The proton of a strong protonic acid (Bronsted) can also initiate cationic polymerization. Its effectiveness depends on the nucleophilicity of the conjugated base,
that is, a low nucleophilicity of the counter ion promotes
polymerization.5 In the case of perchloric acid (HClO4),
the steps of initiation read
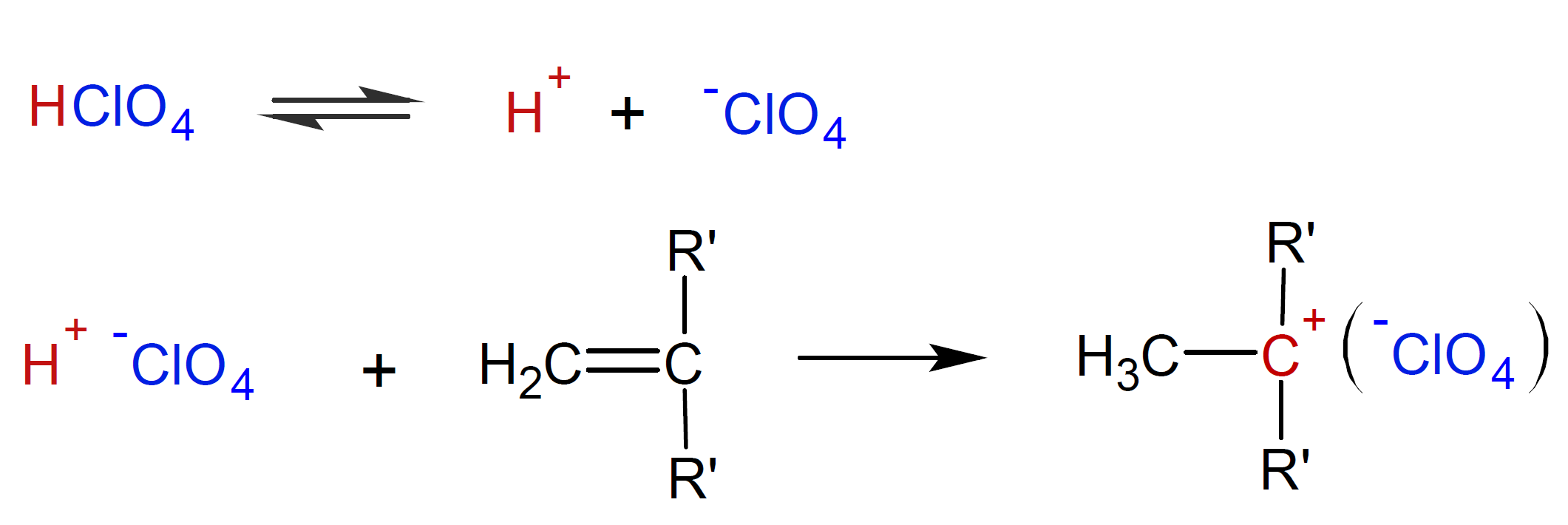
In the propagation step, each carbocation reacts with a vinyl monomer to form a new carbocation:
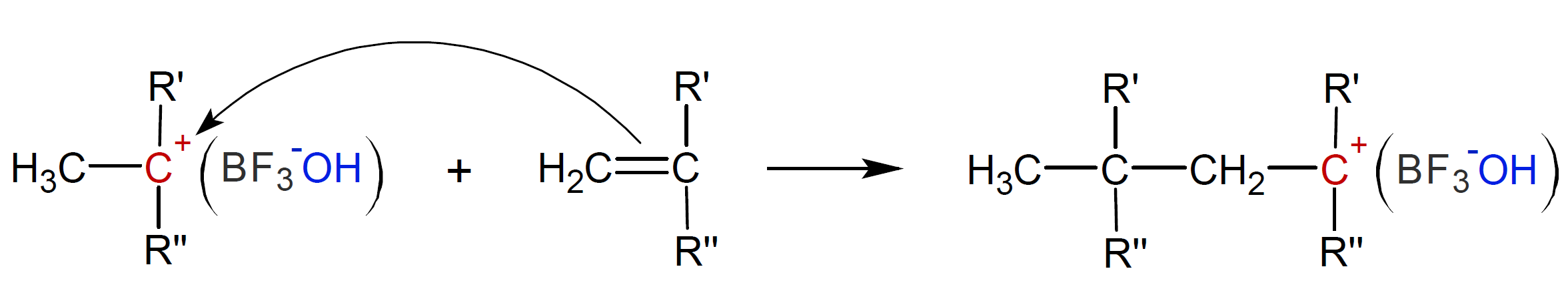
This step repeats itself until all monomers are consumed or until the polymers undergo a termination step. This is the case when the counter (gegen) ion removes a proton leaving an unsaturated terminal unit. The active chain end can also transfer a proton to a monomer, leaving a new cation which can initiate chain growth:1,9
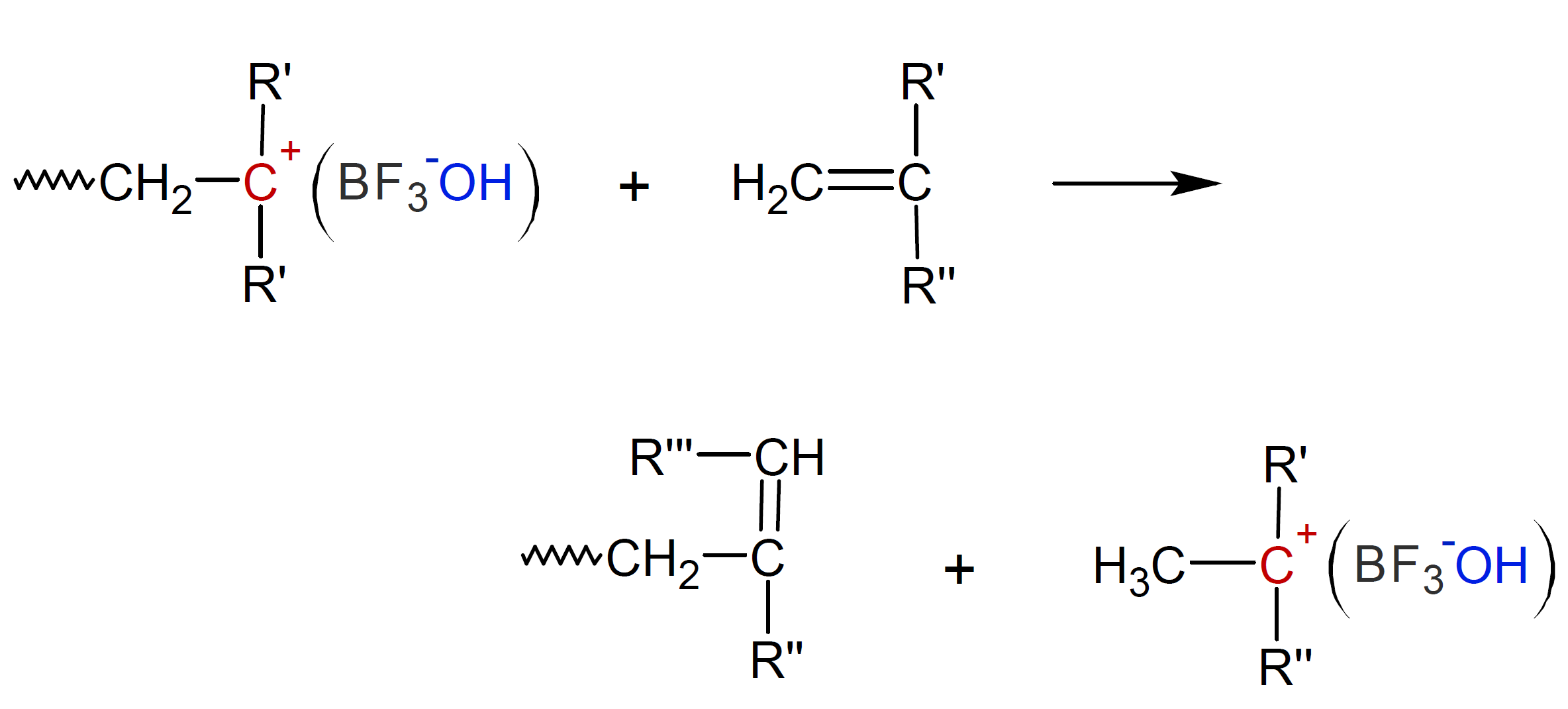
In the case of vinyl ethers, a proton can also be transfered to the counter ion which also terminates the propagation of the carbocation.9
The cationic polymerization usually proceeds at high rates both at high and (very) low temperatures.1 For this reason, a uniform and constant reaction condition cannot be maintained during polymerization. For example, isobutylene at -100°C in the presence of a strong Lewis acid polymerizes to high molecular weight polybutylene within a fraction of a second.1 To prevent excessive rise in temperature in the reaction vessel, an internal refrigerant is usually added to the mixture that dissipates the heat by evaporation of a portion of the liquid.
Both the rate of reaction and the molecular weight decreases with increasing temperature. For this reason, low temperatures are usually preferred. In fact, molecular weights obtained at room temperature are often much lower than those achieved by free-radical polymerization. The rate of reaction also depends on the type of side groups; in gerneral, the more stable the carbocation end group, the greater the rate of propagation. Steric hindrance also effects the rate of polymerization. For example for alkyl vinyl ethers polymerized at about -80°C, the reaction rate decreases in the order:5
methyl > n-butyl > ethyl > i-propyl > t-butyl
For styrenic compounds the situation is more complicated; for para substituents the rate increases with the electron-donating inductive effect (+I) of tehe substituent: OCH3 > CH3 > H, whereas for ortho-substitution, substituents retard the propagation regardless of the type of substituents due to strong steric hindrance.
References & Notes
Paul J. Flory, Principles of Polymer Chemistry, 1st Edition 1953 Cornell University
M. Pitsikalis, Ionic Polymerization; In: Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, 2013
S. Aoshima and S. Kanaoka, Chem. Rev., 109, 5245–5287 (2009)
-
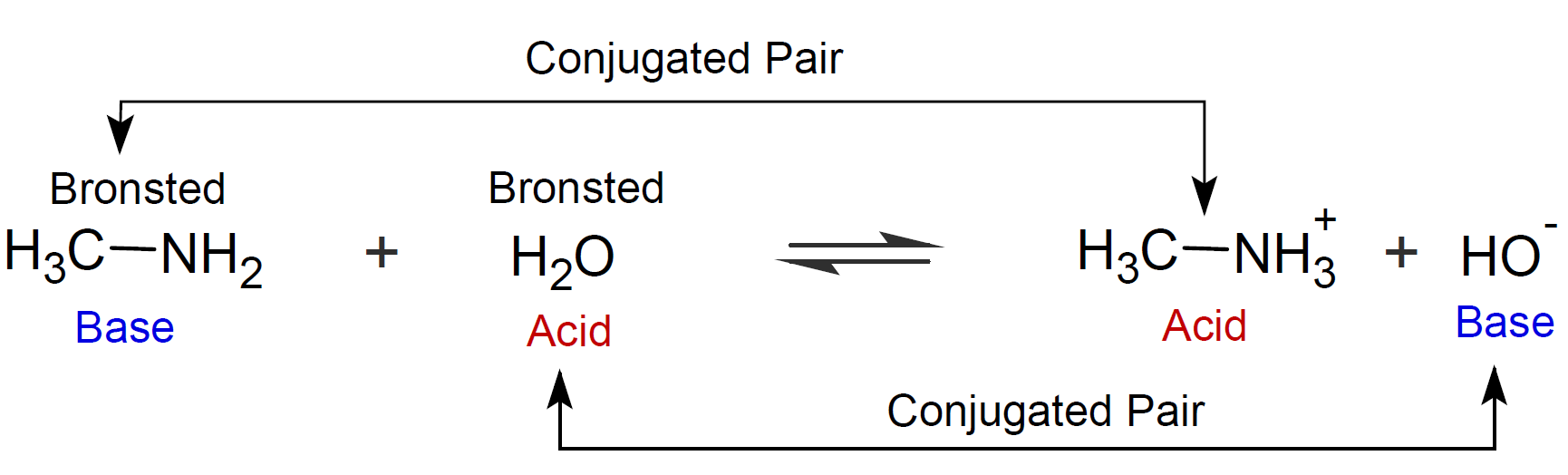
A Bronsted acid is defined as a proton donor and a Bronsted base as a proton acceptor:
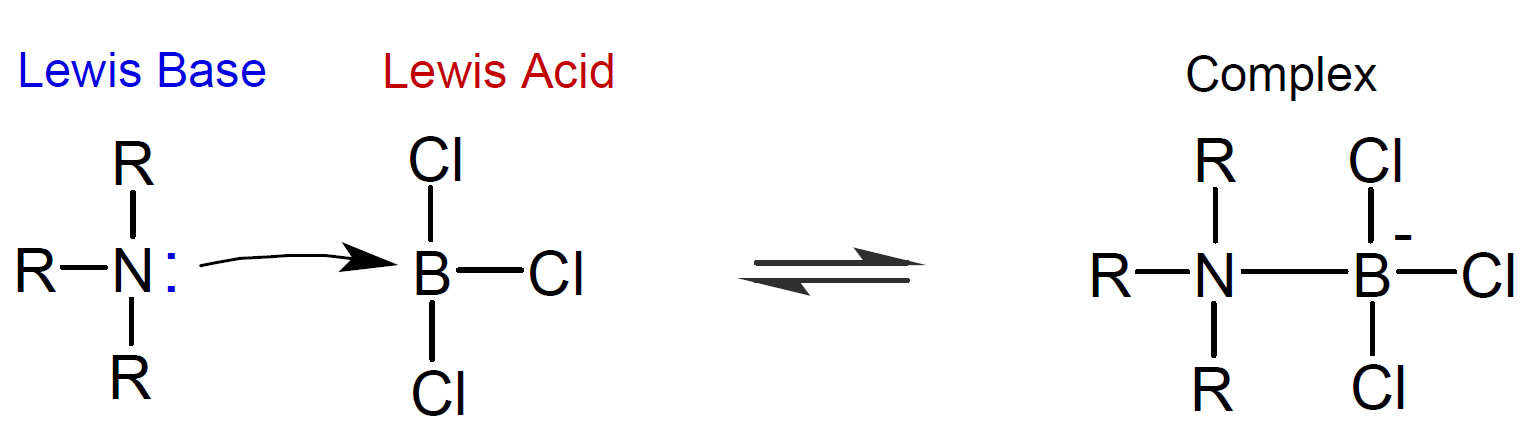
A Lewis acid, on the other hand, is defined as an electron-pair acceptor and a base as an electron-pair donor. Thus, a proton is only one of many species that can function as a Lewis acid:
A. Ravve, Principles of Polymer Chemistry, 2md Ed., Kluwer Academic, New York 2012
-
P.H. Plesch, J.C. Austin, J. Poly. Sci.: Part A: Poly. Chem. 46, 4265–4284 (2008)
-
J.P. Kennedy, J. Poly. Sci.: Part A: Poly. Chem., 37, 2285–2293 (1999)
-
Cationic polymerizations are usually carried out in solvents of low to moderate polarity such as hexane or chloroalkanes,6 which favors solvent-bridged ion-pairs.
-
M.D Lechner, K. Gehrke, E.H. Nordmeier, Makromolekulare Chemie, Birkaeuser, Berlin 1993
-
The strength of a metal ion Lewis acid depends on its electronic properties and structure and generally increases with
- increasing charge density of the central metal atom, for example, TiCl4 > TiCl2.
- increasing atomic number of the metal in each group: Ti > Al > B; Sn > Si
- with decreasing size of the ligands (for small center ion): BF3 > BCl3 > BBr3, TiCl4 > TiBr4
For example, the strength of following Lewis acids have been rated in the order: BF3 > AlBr3 > TiBr4 > BBr3 > SnCl4.5
Revised May 9, 2020